Background
The drug not taken by the patients will obviously not be effective. Consequently, the high discontinuation level (up to 50%) over a 5-year period of tamoxifen outside clinical trials has impact on survival. Sound prospective studies are therefore needed to identify risk factors relating to patients’ non-adherence to tamoxifen. Both these tamoxifen-related topics have become highly relevant due to the recent extension from 5 to 10 years adjuvant tam treatment in clinical guidelines. Our findings will contribute to increasing the quality of clinical follow-up of these patients.
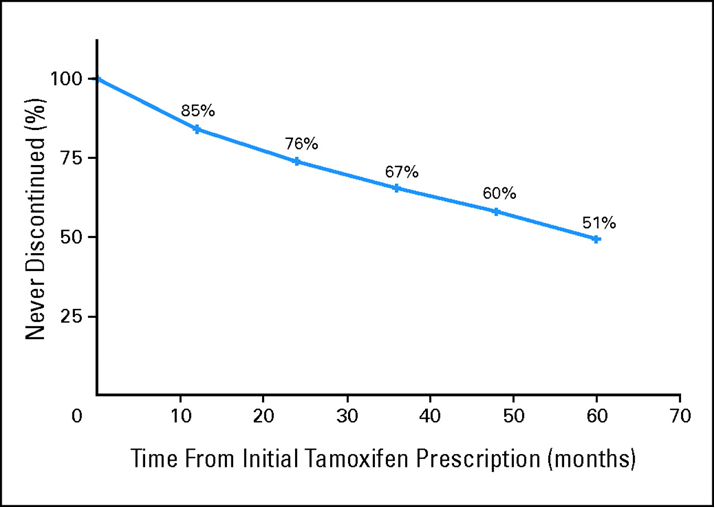
Adherence to tamoxifen outside clinical trials is low. Owusu, C. et al, Journal of Clinical Oncology (2008) 26, 549-555.
Hypotehesis, aims and methods
We hypothesize that the underlying causes of low adherence is complex and consist of both biological (metabolic differences in tam/estradiol levels) and psychosocial factors. We aim to design an algorithm helping clinicians to identify risk factors for low adherence in breast cancer patients in the PBCB-cohort. This algorithm will identify patients with a high risk of becoming non-adherent and make the patient follow-up more focused and individualized.
In this project, 209 patients from Haukeland Unversity Hospital and Stavanger University Hospital areenrolled in a prospective observational study. A number of 10 Patient Reported Outcome Measures (PROM) are prospectively collected to map both health related quality of life and psychosocial factors. The PROMs comprise EORTC-QLQ-C30, EORTC-QLQ-B23, FACT-B) HADS, Uncertainty in Illness (MUIS), fatigue (FACIT-F, VAS-fatigue), General Health Complain, side effects (e.g. joint pain), dietary habits and IBS complaints (ROMA III) questionaires.
Moreover, blood samples from the PBCB biobank will be used to map the metabolite profile in these patients. Furthermore, we have also access to the Norwegian Registry of prescription (Reseptregisteret) to control for use of prescribed drug. In addition to the anti-ER effect, the tamoxifen metabolite profile may also be associated with side effects, specifically to the concentration of tamoxifen and its de-methylated metabolites in serum. This project is part of the PhD projects of Kari Britt Hagen (funded by HUH).
PI: Håvard Søiland, Stavanger University Hospital.
















